























PhoenixDx® SARS-CoV-2 Multiplex IVD (FDA EUA) (50 Tests Per Kit) - TRAXSKU015
TRAXSKU015
Contact us today to get your free validation kit - call 833-548-8378 or email sales@traxconnects.com.
50 Tests Per Kit.
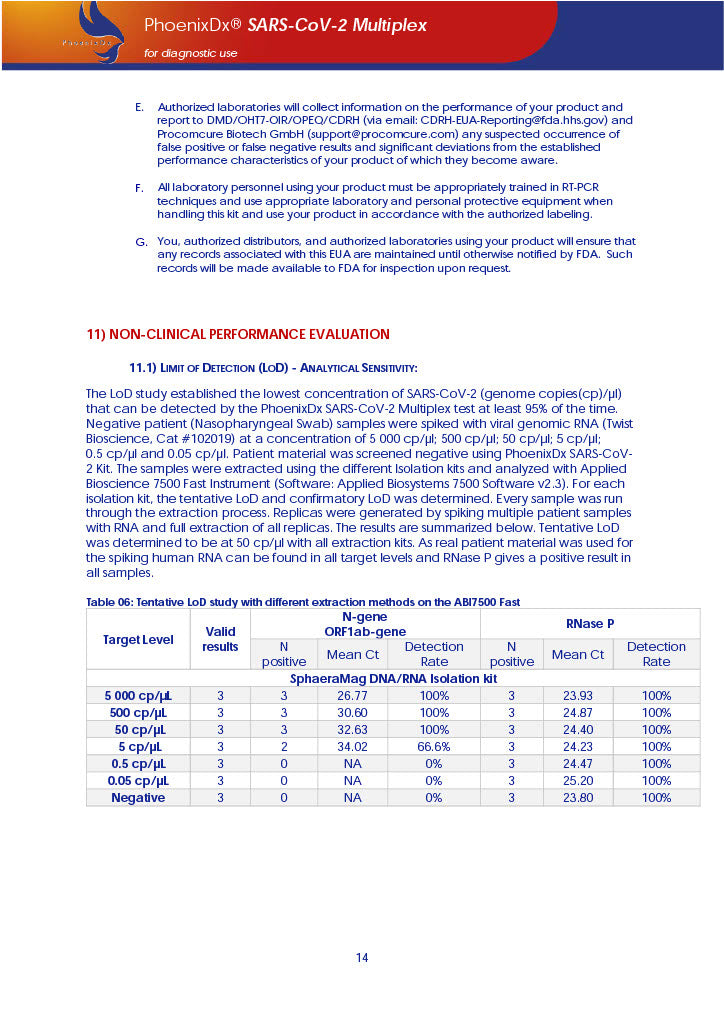
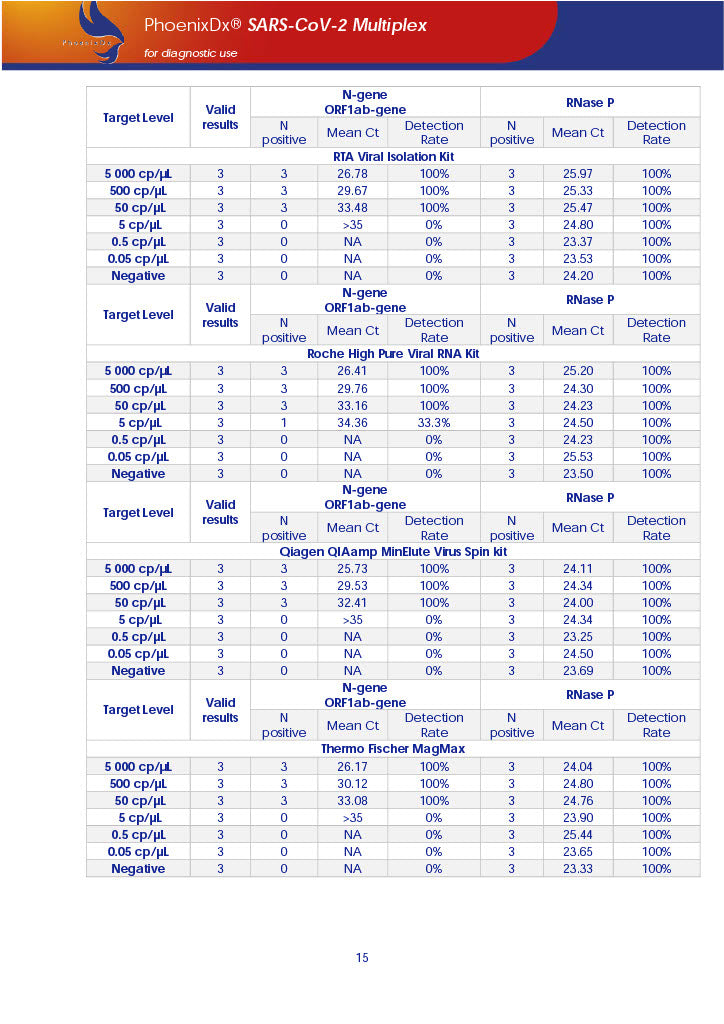
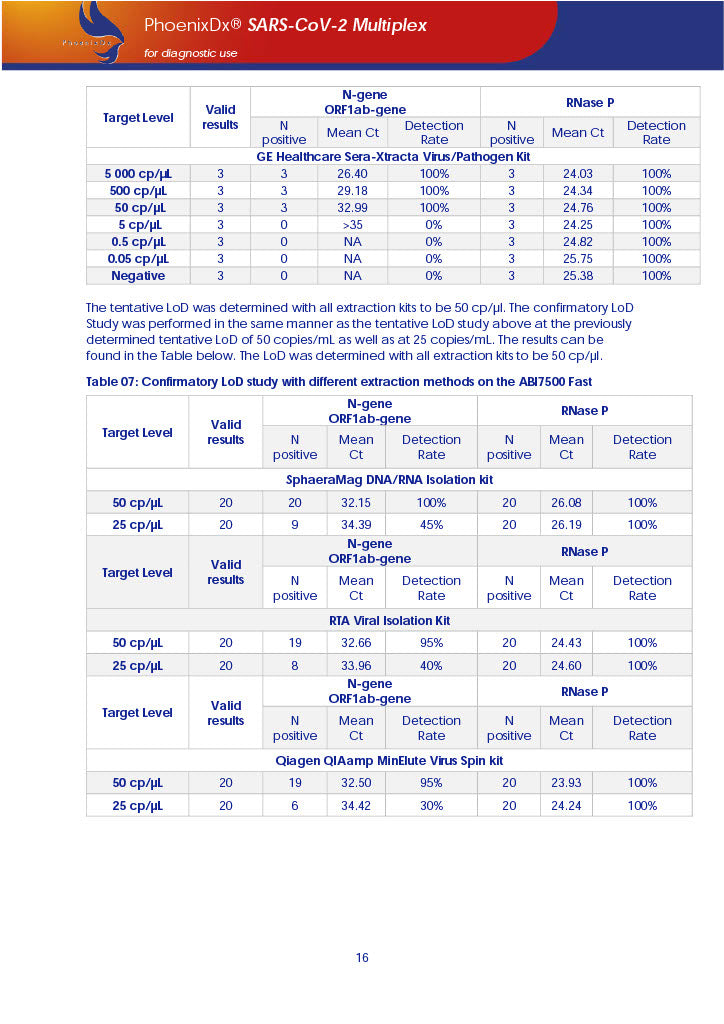
PHOENIXDX® SARS-COV-2 MULTIPLEX (FDA EUA) is a real-time RT-PCR-based diagnostic test for the in vitro qualitative detection of 2019-Novel Coronavirus (SARS-CoV-2) in respiratory specimens and sera from patients who meet COVID-19 clinical and/or epidemiological criteria.
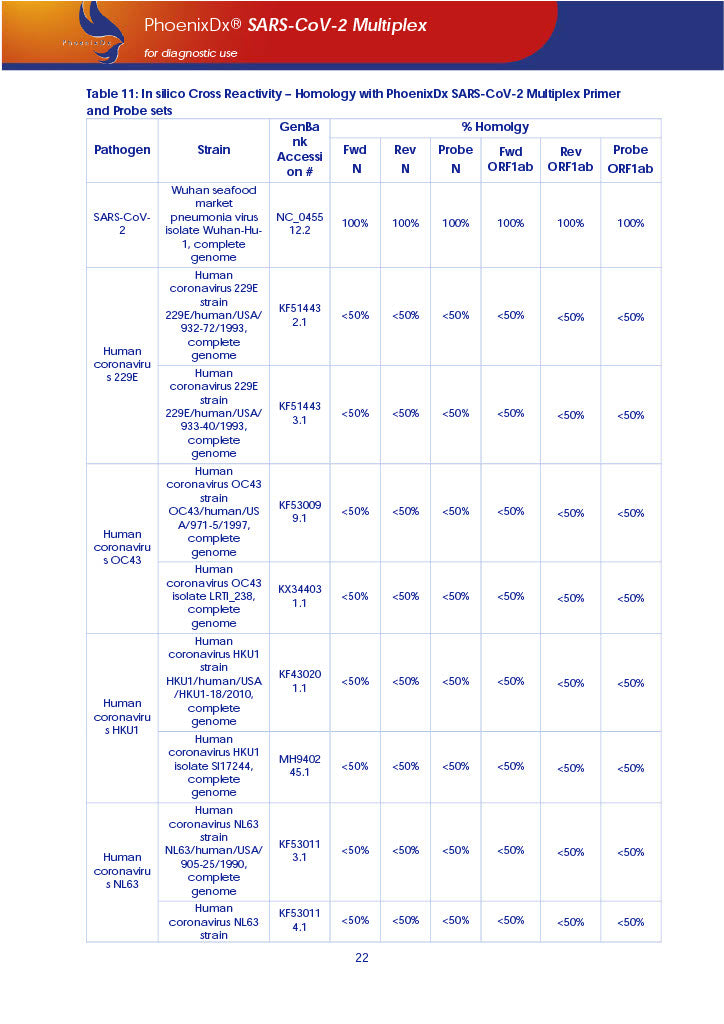
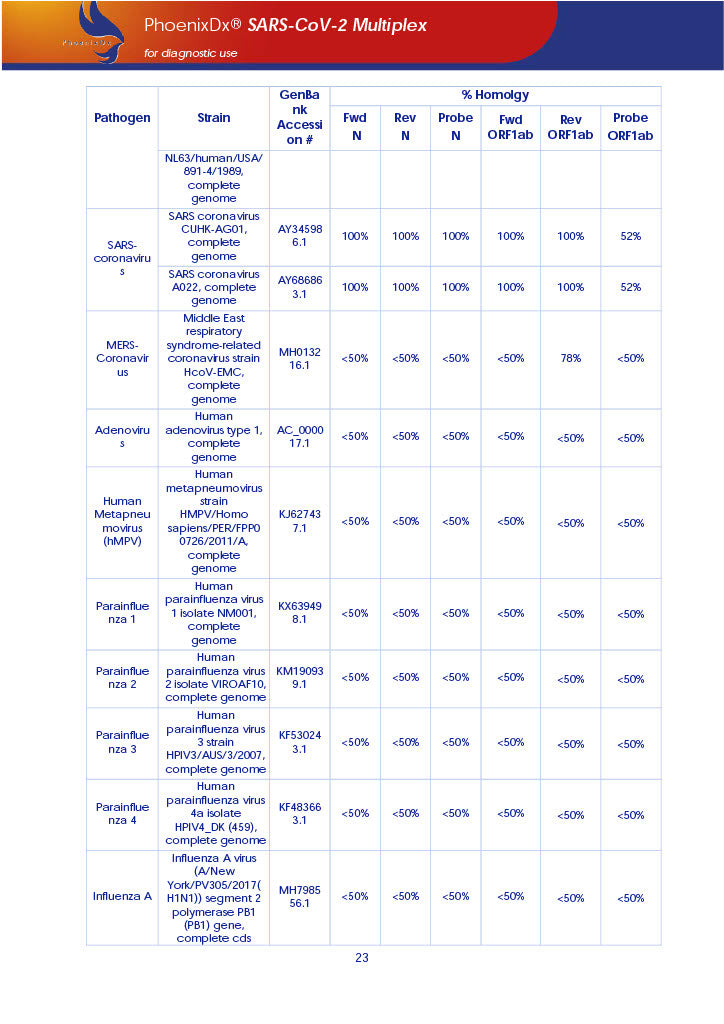
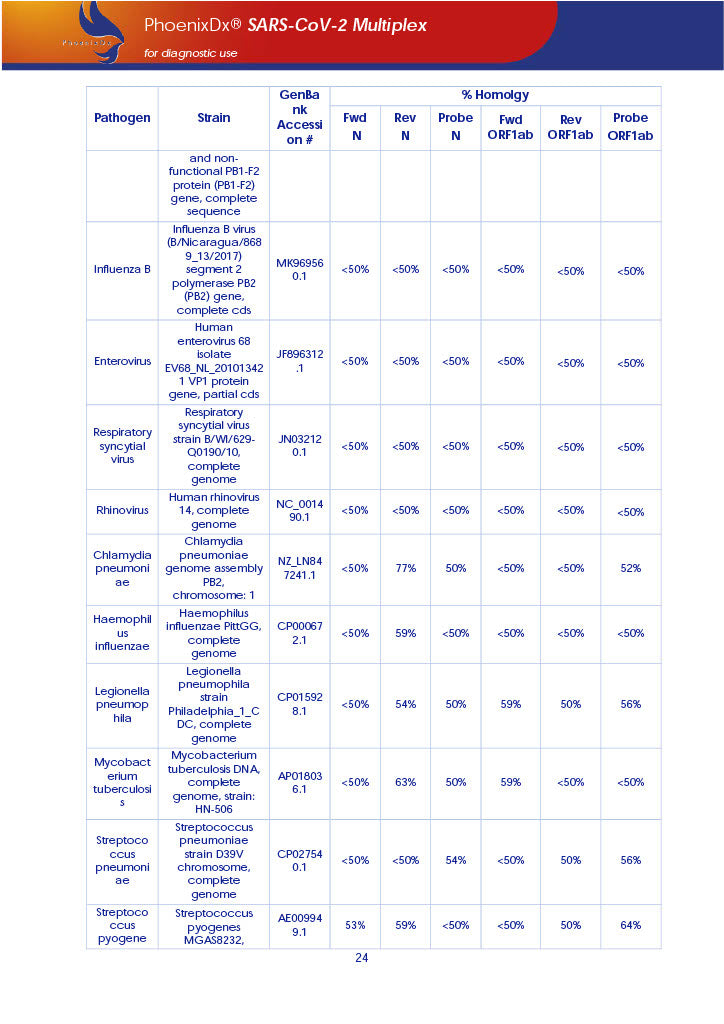
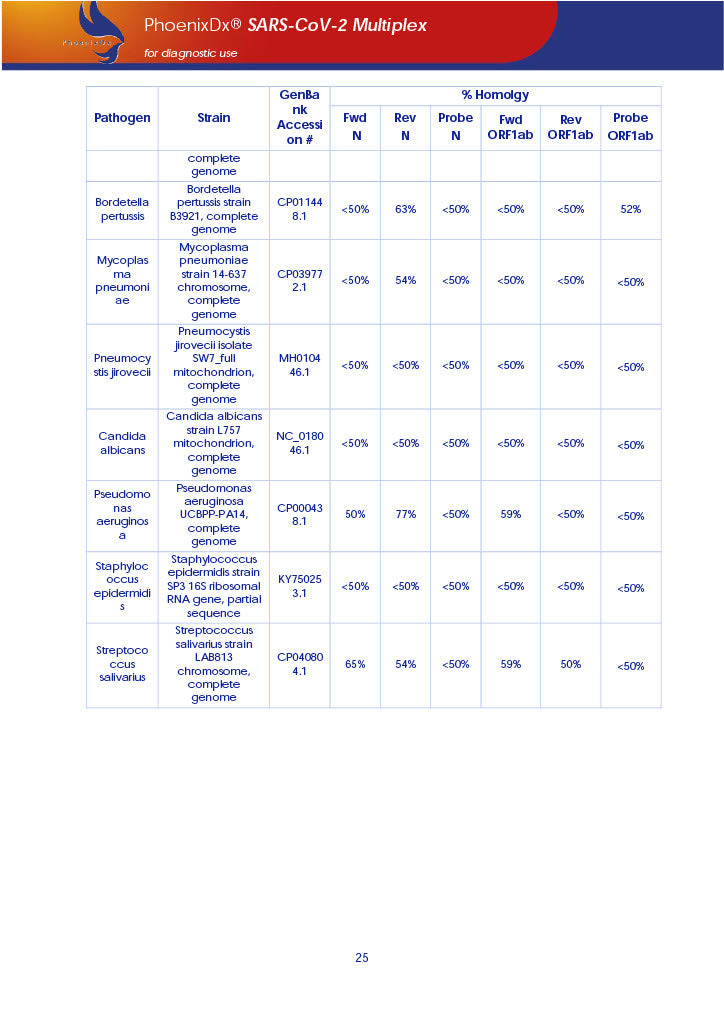
PHOENIXDX® SARS-COV-2 MULTIPLEX IVD detects the presence of 2 different and highly specific gene sequences of corona viruses and one sequence specific for human RNA serving as a human extraction control (HEC). Additionally, a non-infectious target positive control (TPC) is included. The positive control is used to confirm functionality of the assays and overall PCR performance, the human extraction control is to evaluate the quality of the RNA isolation independently from the SARS-CoV-2 assays in a different detection channel.
Need additional payment options for large purchases? We are happy to help, please contact us at 1.833.548.8378 or email sales@traxconnects.com.


